In the Beginning
Darwin developed the theory of evolution to explain the progression of life from simpler to more complex biological forms. In this geology course offered by The Great Courses – How the Earth Works – the professor, Michael Wysession, asked a different, but equally intriguing question, “Where do the component elements of our world come from?”

In order to approach the answer, we have to leave Earth and contemplate the origins of the universe. All cultures have creation stories but without science and the many scientific tools of recent vintage, these stories are missing the details, the nuts and bolts as it were.

It is generally accepted that the universe “began” 13.8 billion years ago with an explosion of unfathomable energy called the Big Bang. Keeping in mind the fact that our Earth is only 4.6 billion years old, what was happening out there in the meanwhile?
Basic Atomic Structure
When I was in college, atomic structure was believed to be fairly straightforward and those concepts are useful for the purposes of this article. The nucleus of an atom is made up of protons which have a positive charge (+1) and neutrons which have no charge. (Despite not having charge, the presence of neutrons is vital in counteracting the repulsive positive force that a group of protons will exhibit towards each other). Orbiting around the nucleus are the negatively charged electrons (-1). The number of protons in a nucleus defines the atomic number as well as the definition of an element which is “all material with the same atomic number.” Through a variety of different physical-chemical bonding types, materials – either pure elements or compounds – needed to have zero charge in order to remain stable, i.e., the same number of electrons and protons.
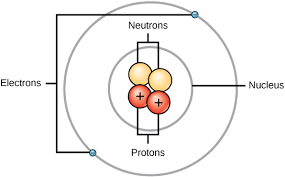
Building with Atoms
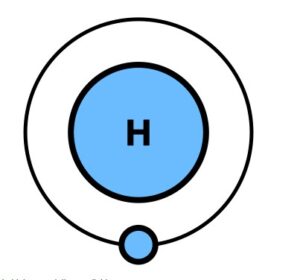
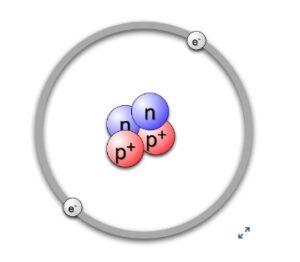
Nucleogenesis, or the formation of elements, began with the Big Bang. In the first 20 minutes after the Big Bang, hydrogen(atomic number 1) and helium (atomic number 2), with some lithium (atomic number 3), beryllium(atomic number 4) and perhaps boron (atomic number 5) were formed. At that point hydrogen and helium were the most abundant material in the universe. Even today approximately 73% of the mass of the visible universe is hydrogen and helium makes up about 25%. The rest of the elements constitute only 2% of the total and they were created inside stars long after the Big Bang.
After only 100 million years, gravity gradually condensed some of the hydrogen and helium into hot rotating masses creating galaxies and stars. However, not all stars are created equal.
For Stars, Size is Destiny
Size is a great determinate of the star lifecycles. Our sun which is of intermediate size is used as the basic yardstick and its measurement is 1 solar mass. Intermediate stars range from one-half to eight solar masses. Low mass stars are a tenth to half of 1 solar mass. High-mass stars weigh in at more than 8 solar masses. The current upper limit in size for high-mass stars is approximately 250 solar masses.
Massive stars have a shorter life span as their cores are hotter and they burn through their hydrogen supply faster. Their entire lifespan may be only a few hundred thousands of years to 30 million years. Our sun, as a midsize star is expected to have a lifespan of 9-10 billion years, although the processes that will make our planet uninhabitable will begin in about a 1 billion years. Low-mass suns are believed to be able to live for trillions of years. Note that this is entirely theoretical since the whole universe is only 13.8 billion years old.
We are Part of the Milky Way

The mass of a star is determined by the amount of primordial material present in the molecular cloud of dust and gas present when gravity exerted its force upon it. In addition, colder molecular clouds generate low-mass stars while warmer clouds give rise to a variety of larger star sizes. It is estimated that our own Milky Way contains about 6,000 molecular clouds with masses of more than 100,000 solar masses.
Stars ignite when their large rotating masses reach approximately 1 million degrees K (Kelvin) created by the fusion of hydrogen into helium. In fact, until the first stars ignited, the universe was dark. “Let there be light” is not just a metaphor.
The Rest of the Elements
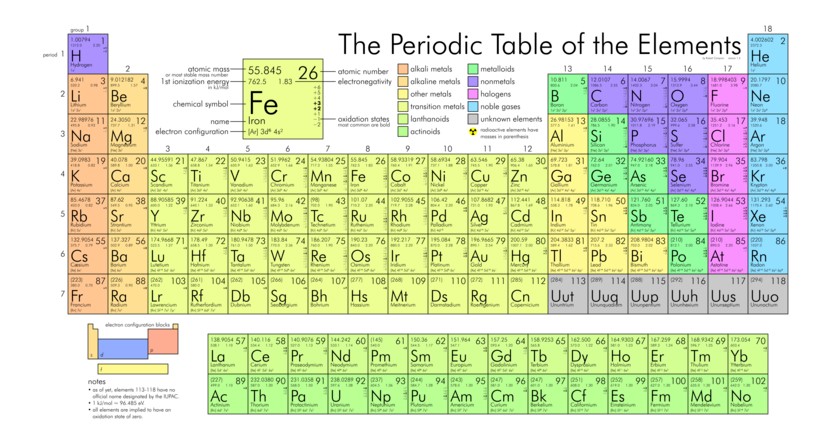
What we have learned is that our world is not a first-order creation. None of our vital elements were formed immediately after the Big Bang and so we seem to be second-order creations. Carl Sagan was absolutely correct when he said that “we are made of star stuff!”


If you have enjoyed this blog, you may be interested in my book “A Habit of Seeing: Journeys in Natural Science:
Very nice recap and information. Thank you. luis
It is always a pleasure and a breath of fresh air to read your thoughtful scientific systematic methodologies based on evidence and personal knowledge! Thank You Adrienne V
as always, have enjoyed your simplified presentation of these complex items.
please proceed with it.
elinechama
Thank you. It was a pleasure. Esther