Nature is never simple. We have discussed chlorophyll, the chlorophyll containing structures known as chloroplasts, and even animals that have incorporated chloroplasts into their cells. Today, we will move along to other pigments found in plants.
Accessory Pigments - Carotenoids & Anthocyanins
Carotenoids
The carotenoids are divided into two classes based on their chemistry – carotenes and xanthophylls
Carotenes are familiar to us as the orange pigment in carrots, potatoes and cantaloupe. Indeed, our English word for carrot is the same as the Latin carota. Carotene is responsible for the orange color in fall leaves.


The word xanthophyll comes from the Greek xanthos meaning “yellow” and phyllon meaning “leaf. It is primarily responsible for the yellow color of fall foliage and flowers. There are numerous xanthophyll compounds and they account for the yellow color of corn, saffron and even chicken eggs. Xanthophylls are responsible for the yellows in fall leaves.
Caretenoids appear yellow-orange since they absorb light in the blue/green wavelengths reflecting back the orange/yellow wavelengths. . Production of carotenoids begins in the spring. Since that production is not dependent on the level of sunlight, it continues into the fall.
So, why don’t most leaves have a yellow or orange tint during the growing season? Simply stated, it is because their colors are masked by chlorophyll pigment which begins production around the same time in the spring. The yellow or orange color only becomes visible in the fall as the chlorophyll is resorbed by the trees. If, however, there is a superabundance of carotenoids, the leaves may stay a yellower-green.


Oil and water do not mix. Therefore, carotenoids, being fat-soluble, cannot dissolve into the watery gel inside plant cells. Instead, they attach to cellular proteins or chloroplast membranes, the chloroplasts being the plant organelle in which photosynthesis takes place.
Carotenoids have two seemingly contradictory functions. One the one hand they absorb light in the range where chlorophyll does not. Its two peaks are around 450 and 475 nanometers which includes violet and blue-green light. The positioning of these molecules near the chlorophyll molecules allows transfer of electrons into the photosynthetic pathway through the chlorophyll molecules. This expands the efficient use of sunlight energy. This is particularly useful under conditions when sunlight is somewhat muted.

However, when light is very strong, or, if there are sudden surges of light through openings in the leaf canopy as the sun travels through the sky, the chlorophyll can become temporarily overloaded and the leaves can become damaged. This excess energy can be dissipated better by transfer back to the carotenoids which then dissipates the energy as heat thus protecting both the photosynthetic mechanism and the plant leaves. Parenthetically, light in the ultraviolet b range (280-315 nm) which plants do not use for photosynthesis and is damaging to human skin can also damage plants. Plants manufacture sunscreen chemicals to protect themselves.
Anthocyanins
This plant chemical is a flavonoid, and its production is light dependent. In contrast to carotenoids it is water soluble and found in the vacuoles of the cells
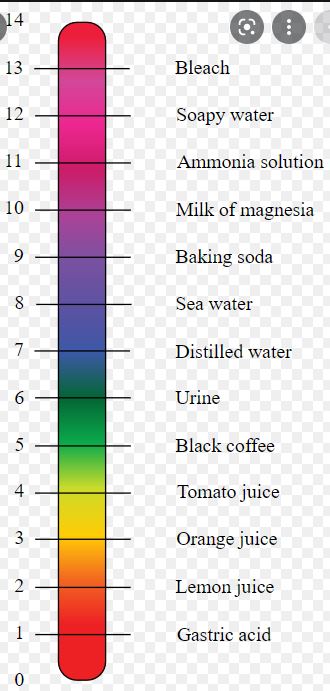
Depending on pH, the color generated may be red, blue, purple or magenta depending on soil pH. As a yardstick, something that is totally neutral – neither acidic nor alkaline has a pH of 7. Distilled water has a pH of 7. In regard to leaves, soil pH less than 3 yields red, 7-8 produces violet and above 11, we get blue.

Anthocyanins are not usually produced in the leaf until fall when it is formed from sugars in the leaf. Yes, there are red-leafed tree cultivars such as Japanese maples. Additionally, Some young leaves also will show a reddish tinge in the spring . There is some suggestion that the pigment protects the leaves from damage if there is unexpected cold weather. In those conditions when the leaf matures further and is making enough chlorophyll, the anthocyanins are degraded and disappear.
Anthocyanins are also responsible for the red color found in cranberries, blueberries and plums



Cool nighttime temperatures followed by dry but warm sunny days promote the production of anthocyanins. Trees with red fall displays include maples (Family Sapindaceae, Genus Acer), oaks (Family Fagaceae, Genus Quercus)), dogwoods (Family Cornaceae, Genus Cornus) and sourwood (Family Ericaceae, Genus Oxydendron).
One last installment will be uploaded finally to finish this series.
Once again, many thanks to Howard Neufeld, Appalachian State University.
If you are enjoying this series on chlorophyll and other pigments, you will enjoy my book, A Habit of Seeing: Journeys in Natural Science.

Thanks for the free sample of your next book.
Do U know about BronxParksSpeakUpXXViii? I think the Gingko is alive. Time will fly.